Behind the Study is a series of transcribed videos from researchers elaborating on their oncology-focused studies published by Oncotarget. A new Behind the Study is released each Monday. Visit the Oncotarget YouTube channel for more insights from outstanding authors.
—
Dr. Yves Chabu from the Department of Biological Sciences at the University of Missouri describes a research paper he co-authored in 2020 that was published by Oncotarget, entitled, “Evaluations of CRC2631 toxicity, tumor colonization, and genetic stability in the TRAMP prostate cancer model.”
Dr. Yves Chabu
My name is Yves, Yves Chiswili Chabu, and I’m a professor in Biological Sciences here in the University of Missouri, Columbia. And to answer the question, “How did we get to the story that we recently published in Oncotarget?” When we first started, we really started from the concept that in clinics, one of the significant challenges is to identify therapeutics that are highly selective. So, by that, I mean having an agent that can selectively kill the cancer cells without killing their normal cells. So, a lot of these cancer therapeutics, especially when you’re talking about chemotherapies, they not only kill the cancer cell, but also kill the normal cells. So this can generate considerable toxicities and morbidities for the patient. So, we started from a sort of need to come up with a different way to target these cancer cells. But also to do it in a way that does not necessarily require enough theory understanding of the molecular landscape or the molecular drivers of that cancers.
Let me just take a step back.
We now know with the precision medicine approach, one can obtain biopsies from the patients and then from those biopsies, define the molecular landscape or at least identify genes that are driving the cancer. And that knowledge will then inform the physician’s decision as to which therapy is better suited for that patient. So that’s your precision medicine space. That has led to significant improvements in terms of patient outcome, but what we wanted to do was to also think about ways that are not that selective. In other words, to find the strategies that can target cancers more broadly.
Now, about a century ago or more, there was this realization that bacteria can target cancer cells, and we had already some data showing that this specific Salmonella strain that we had could target cancer cells – basically killing cancer cells, but leaving the normal cell largely unaffected. So, we then reasoned that maybe we can utilize that and go into an in vivo model to really test the efficacy of that approach or how good that approach was. We turned to prostate cancer because that’s where we had most of this data, right? So, we turned to the prostate cancer and there’s a mouse prostate cancer model. As you probably know this, prostate cancers can develop resistance to treatments and the therapeutic landscape, but that indication is limited. Immunotherapy also doesn’t work there and hormone therapy eventually leads to a relapse for those patients.
So we took that specific indication, meaning that specific cancer type, and asked, “Does this bacteria that shows tumor tropism in vitro, that shows cell activity in vitro, can it also generate similar effect in an animal model?” That was really where we started. That’s really the initial question that we went after. During the course of the study what was really unexpected was that although we saw these targeting, so we can see the biologic bacteria homing into the cancer tissue very specifically. So they are colonizing the cancer tissues, and not only are they colonizing the cancer tissue, but there are staying there. They’re staying there for at least a couple days, and that finding is relevant because normally this bacteria get cleared away really fast in the host. But what we saw is that it was able to go into the cancer tissue and stay in the cancer tissue. That is really important, right? So, you can now have the bacteria that is going to the tumor, and then it is colonizing. That means they have to divide in these tumors and evade clearance.

That was really encouraging for us, right? Then we asked, “If the bacteria is staying in the tissue, can we…” Let me put it this another way: So, here the bacteria is staying in the tissue – you also want to make sure that the bacteria itself doesn’t accumulate additional genetic mutation that will make it lose its characteristics – and the characteristics that I have in mind here is really the safety. We had shown that the bacteria itself was quite safe in this animal model, so we didn’t see some really significant toxicities in these mice, and we had also evaluated it in dogs where it was well tolerated. So, what you want then is to have a bacteria. I’ll use the word biologic and bacteria almost interchangeably. But what you want is that not only you’re targeting the cancer tissue and it is stable in the cancer environment, but you want to make sure that bacteria doesn’t accumulate additional genetic lesion, such that it loses all its characteristics, i.e., its ability to persist into a micro environment and to kill off the cancer cells.
So then what we decided to do was, “OK, let’s then evaluate its genomic stability.” And in so doing, we could see clearly that it was also genomically stable. Then we had a series of characteristics and, quite frankly, quite attractive characteristics. We had a biologic that was safe and in addition to that, it was able to target the cancer tissue. And three, it was genetically stable even after it colonized the tissue for days and weeks. That was very encouraging. Then we asked, “What are the consequences of these targeting effects? What are the consequences of having the biologic going into the cancer cells?” And one area that we really wanted to focus on is the ability of these cancer cells to now release immunogenic molecules. So, you want the cancer cell to release molecules that can now attract the immune cells to the tumor micro environment.
Why is that important? It is important, especially in the case of prostate cancer, because the prostate cancer environment is immunosuppressive. So, prostate cancer has enact mechanism to suppress immune cells and exclude, really, immune cells from the cancer tissue. And because of that, even your immunotherapeutics that have shown great results in say, skin cancers, they don’t work on the prostate. For prostate cancer, there is a need for strategies that can remove that immune barrier so that these immunotherapeutics can actually work in that setting. So we then reasoned, “We now have a bacteria that we know is immunogenic, right? It’s a bacteria after all. And it goes to the tumor, colonize the tumor and then it is genetically stable in that tumor.” Now we will go and say, “Test the hypothesis that these bacteria can also have an immunogenic effect.”
That is, it can cause this cancer cell to release all these chemokine into the tumor micro environment and in so doing, recruits immune components in the cancer cells. And it did. So that was really satisfying. We could see from those experiments that animals that were treated with the bacteria, again, the prostate cancer mouse model, their cancers had a lot more infiltration of immune cells. Right. So, you had immune components in the tumor micro environment and there were there in a higher frequency or quantity, if you will, than the control mice that were just treated with the buffer. So we thought, “This is great, right? We are getting an immune stimulation. We are recruiting immune components now in the tumor tissue. If that’s the case, we should expect to see an increase of cancer killing immune cells as well.”
So we saw a series of different type of immune cells in the cancer microenvironment. So, CD4 positive cells, which are required and critical to activate the cytotoxic CD8 positive cells. So, we saw an increase of CD4 positive cells in the cancer microenvironment. Then, we asked if we could also see these CD8 positive or cytotoxic T-cells, and unfortunately, whatever we saw there was really transient. So we’ll see some CD8 positive cells, but then rapidly, those numbers will basically all just would dwindle down and you get a decrease. It really hinted that perhaps what is happening, the cancer cells are deactivating these CD8 positive cells. So they are deactivating the cytotoxic T-cells, and one mechanism is essentially through these Citi PD-L and PD-1 mechanism.
So we then thought if we could couple the use of the bacteria with blockades of the signaling axis, so that is, we’re now preventing cancer cells from inhibiting CD8 T-cells, we will see a decrease in disease burden. So that was the hypothesis that we went in. It was very rewarding to see that indeed, when we couple now these immunogenic and tumor targeted bacteria with antibodies that block the PD-1, PD-L signaling axis, we could now decrease disease burden in these animals.
I think that’s the novelty of the paper. So that is to use something that one wouldn’t even consider using, right? So you’re using a genetically attenuated bacteria, nonetheless, but it’s still a bacteria, and utilizing its immunogenic abilities and engage immune components and activate the sense of fighting immune cells definitely has some merit. And in this case, we could actually see a decrease in disease burden in these mice.

For us, it was really proof of principle that one can use these immunogenic biologic to now break down that immune barrier we talked about at the beginning and allow the immune components or the immune cells or the host’s immune cells to surprise or to kill off the cancers. So it’s a proof of principle. My hope is that the rest of the fields and all the other researchers out there, or the community will manage to get past this concept that it is a bacteria and really see how you can better functionalize it to achieve better clinical outcomes because that’s why we’re all into this. We’re all into this to help our patients. So if we can come up with strategies that can leverage these characteristics of this biologic to indeed generate some durable, clinical benefits for patient, then it’s a win-win situation.
What is next? So what will be the next chapter? I really hope this happens that the entire community picks this up and try to see better ways of functionalizing it, especially a better outcome. Another benefits of this biologic. So you can use it as a tumor immune activating agent, but you can also use it as a vehicle. So you can envision leveraging its tumor targeting capacity to deliver therapeutic loads into cancers, and so you can pack therapeutics into the cells and you can do this. It doesn’t have to be small molecules, but there’s a thing that can be genetically engineered in the bacteria and be delivered specifically, at least with preference, preferentially delivered into the tumor tissues. So that is also, I think, a clear application for this biologic.
I think I should also note that there is a potential here to expand this to other cancer types where immunotherapy does not work. And here, we have started looking at pancreatic cancer because pancreatic cancer has the same limitation, meaning that it’s in highly immunologically suppressed environment. These are considered immunologically cold-environment cancers. So we are now using that in that indication in pancreatic cancers, and we are seeing really beautiful responses in the host. We’re doing this in mice, but we can see from the spleen, you can see nice activation of immune components in animals that are both. They have the cancer and treated with these bacteria. So you can see an elevation of these CD8 cells in the spleen. Just like in a prostate cancer study, we see that it does not always translate.
The splenic activation that we’re seeing does not necessarily translate into a durable colonization of the tumor by the CD8 positive cells, so it’s similar to what we saw in the prostate. It’s a transient colonization. And then I think you’ll have these suppressive mechanisms that are enacted by the cancer to keep the immune cells away. But now you can imagine, even for that specific indication, coupling, checkpoint blockades, or immunotherapy with the biologic, which has now also has been a surprise. At least that’s the hypothesis that we are pursuing. We can now suppress these really deadly… and again, the therapeutic landscape with that indication is really meager and needing additional agents. So we would like to see that maybe these biologic, this bacteria coupled with the immunotherapy will generate some benefits in animal models. And here, we’re talking about just shrinking all the cancers and ideally, extending the more life.
So those are the sort of the spaces where I can see opportunities. Again, the pancreatic cancer space we’re definitely exploring opportunity for this biologic in that context, but I really hope that the entire community can leverage some of the characteristics of this bacteria. It will help out our patients. Now I should also say that it is not that wild a concept. People have used oncolytic viruses and in fact, there are trials. People using oncolytic viruses to essentially generate immune response and therefore kill, eliminate the cancers. So here it’s a similar approach. Just like people are using oncolytic viruses in their clinical trials and clinical studies, here, we’re using a bacteria and it’s generating some killing locally but also activating the release of these immunogenic factors, which then recruits immune cells to the cancer.
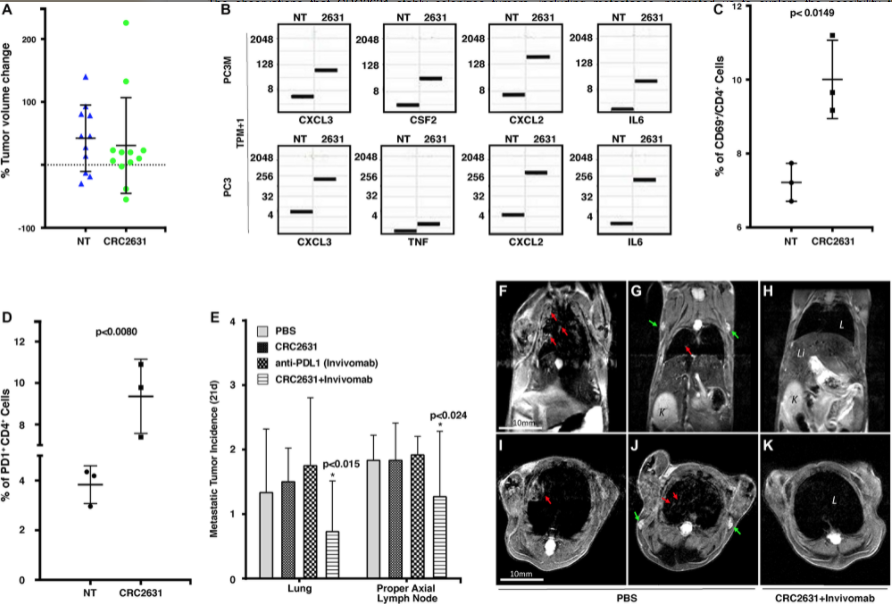
So I think it’s definitely a good proof of principle. It’s a good place to start. It is a good place to be, and then I think further development is needed to really even further refine this technology and see if we can evaluate more broadly across different types of cancers. So we are using it in immunotherapy setting, but we also have some additional data in the context of chemotherapy. We’re seeing some really interesting interaction between chemotherapies and hormone therapies, also in the prostate cancer space. So chemotherapy, hormone therapy and immunotherapy in a prostate cancer space, there is some clear interaction between these agents and those therapeutics.
I think it’s very important to mention. I don’t think I could say it in more stronger terms, but I’m a big proponent of collaborative research. And this work will not have been possible without the collaboration. And in fact, if you look on the manuscript, it’s people across institutions.
So we have researchers like at Yale that performed some of these immunological profiling or this profiling the immune landscapes in all these mice. And we also have my colleagues here at MU that played an important role in modeling, right? So you see, we actually integrated all these different aspects of research, so we had immunologists contributing. We have people that are remodeling all these genomic data that we had. So all that came together to make this story possible. So again, I really think that this manuscript really exemplifies the utility and the value of collaborative research across different disciplines. Again, it was a genomics, it was some of the medical modeling and we had basic cell biology. And then you will also have people in animal science, helping with this and this. People in radiology and radio imaging helping with it.
So it was really team efforts, where everybody brought to the table some unique aspects of their strength. And that’s how this paper really came into being.
I want to thank every single one of my collaborators and people in the lab. I want to thank Bakul, a coauthor on the paper for their hard work. I want to thank Rob, who’s also author on that paper. And again, they worked tirelessly. So there was a post-doc and they worked tirelessly to really make sure that everything was done to the highest standards. So that was really great. That was fantastic.
And of course, I want to thank all my colleagues on the manuscript. Elke Gulden at Yale and our imaging facility here. It was really a team effort. And Dr. Stober also for his modeling on the manuscript.
But that was really fantastic and we had a lot of fun with it. The modeling really started as a casual conversation and it really evolved, and it was something that we started over the phone and then, so Dr. Stober and myself, our families play soccer together. So we will play soccer and then at the end of the soccer, we start talking about these concepts, how we can model this. And then we followed up with phone conversations and again, with more soccer and the rest is history. So it was really nice. It was a really pleasant.
Click here to read the full study published by Oncotarget.
YOU MAY ALSO LIKE: More Oncotarget Videos on LabTube
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared freely with friends, neighbors, colleagues, and other researchers.
For media inquiries, please contact media@impactjournals.com.
