Dr. Mobin Karimi from Upstate Medical University at Syracuse describes the research behind a 2020 paper he co-authored that was published by Oncotarget, entitled, “CrkL is required for donor T cell migration to GvHD target organs.”
Behind the Study is a series of transcribed videos from researchers elaborating on their oncology-focused studies published by Oncotarget. Visit the Oncotarget YouTube channel for more insights from outstanding authors.
—
Hi, my name is Mobin Karimi. I am an assistant professor at the Department of Microbiology and Immunology at the Upstate Medical University at Syracuse, New York. Research in my lab is focused on the cell transduction pathway that include T cells, B cells, and NK cells, ILCs. More specifically, we are interested in modulating signal, transduction pathway downstream. And the read-out of this we presented is a disease model, could be graft versus host disease, graft versus tumor, and also autoimmune models, and you can apply to many, many different things. We work as an upside down pyramid. We are looking at the phenotype on the animal models, and then step down, we look at the cell function and we are looking at the transcription factors and we are looking at specific genes and we can target to specific genes and then see these genes are responsible for this phenotype.
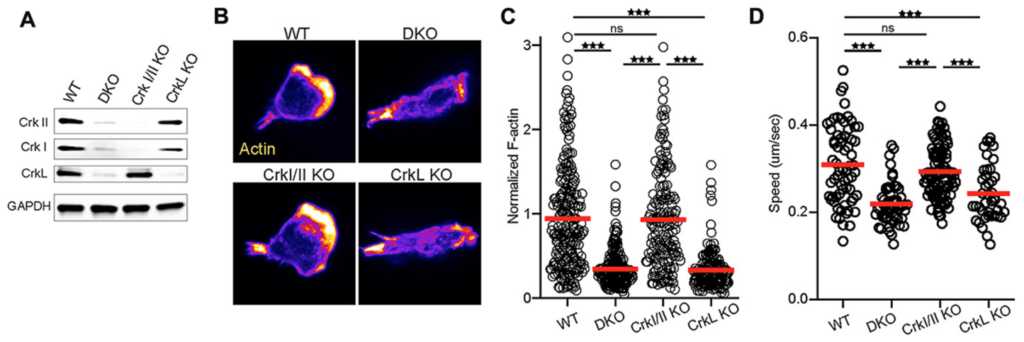
We would like to think … the paper that we submitted for Oncotarget, that we are very happy with them and we are surprised by many aspects of them because when we modulate the chemokine receptors and the migration pathway, we want to look at how these T cells will migrate to the inflamed tissues. For example, in a normal circumstances, the T cells might not migrate from one area to another if you modulate some of the chemokine receptors. So in this manuscript, we looked at three different genes. One was Crk1 as a part of CT10 regulatory kinase pathway, and also we look at Crk1 knockout, Crk double knock, which means that Crk and the ligand for Crk is also knocked out. And we also specifically looked for ligand, what happened if you knock out the ligand. So, in vitro and in vivo, we saw a little bit of a difference in the migration, but that did not give us full satisfaction, because we wanted to test this in disease models.
So in this manuscript, what we did is we took mice and gave them full-body irradiation, and then did a major mismatch transplantation from B6 mice, which genetically, they are different. What happened is that the wild-type cells were able to migrate from secondary lymphoid organ to liver and small intestine and skin, which are the GvHD-target organs. But the mice with the Crk double knockout and Crk ligand knockout were unable to migrate to GvHD-target organ. What happened is when we irradiate mice and those mice produce cytokines in their inflamed tissue. So the donor T cells will come and they will migrate to those inflamed tissue and they generate severe damage. And donor T cells trafficking is now one of the hallmark of GvHD. We were so surprised that we did an Allo mismatch, allogeneic T cells, they were unable to travel to the GvHD-target organ.
We quantify those, and quantification data suggested only 10% of the cells will be able to migrate versus, in the wild-type, they were like 90%, 80%. So that was significant for us. So when we checked with this mice will develop graft versus host disease, since the migration was defected significantly, the host mice did not develop graft versus host disease.
And then we checked with a clear tumor. So in the next experiment what we did is we irradiate them, we establish the Allo mismatch transplant model, and we also give them B cell leukemia. We were so surprised that this donor T cells, either from the wild-type or from the knockout, they were both able to clear the tumor in the periphery, but they did not cause GvHD.
So we wanted to see what will be the limitation of this model. The limitation of this model is to check is where we establish GvHD. But instead of give them IV tumor, we give them IP tumor under the skin and see if the donor cells can migrate under the skin and can clear the tumor. So in the wild-type models, donor T cells were able, you go to secondary lymphoid organs, go to liver, small intestine, also to tumors under the skin and they were able to clear the clear it. But when we look at the Crk knockout and CrkL knockout, or the double knockout, they were unable to go to the tumor where you put in under the skin.
So in hematological malignancies and since everything takes place in the periphery, it would be a good model to use. But for the solid tumor, this might not be a useful tool. So we looked at both, whether it can be applied to hematological malignancy, whether it can be applied to solid tumors. In our lab, we wanted to research, look at all aspect of the models. We wanted to look at the limitation of it, how much we can push this to make it a better model. And this data showed that in hematological malignancy, donor T cells have a defect in the migration and they cannot go to GvHD-target organs. But they can clear the tumor in the periphery, and they do not cause GvHD. So as far as graft versus host disease, it’ll be a great model.
Our collaborators and other people are working to make this as a small molecule and they can inhibit this, and hopefully we can start soon in the clinical model to move it from the bench side to the bed. And we will also check this and see what specific pathway is disrupted in the mice and what specific other pathway is disrupted in the human as well.
So at this point, we are satisfied with our in vivo mice model, but we wanted to test this also in the human models and see if there is any similar effect that we see the migration. And if that’s true, hopefully the small molecule will be an ideal candidate for hematological malignancy, and also for graft versus host disease, because graft versus host disease is one of the diseases, but there are many diseases involved that cause autoimmunity, primarily by T cells. And if you can inhibit T-cell migrations to inflamed tissue, that might be used for other diseases like arthritis, other autoimmune models. And so really excited about this and we are hoping that we can move this forward as far as we can get.
Other projects in my lab is we studied IKK signaling. We have recently developed a small inhibitor, and when we looked at in mouse model, that if you inhibit IKK signaling and upregulate some of the molecules that are responsible for the cytotoxic function, but it inhibits cytokines productions and chemokine receptor upregulation. So in the lab recently, we are moving this to how that will affect patient samples. That patients who developed GvHD or have autoimmune disease where their T cells are inflamed, how can we find out which one will be a better model.
And second model that we are using this ITK models for is there are a lot of drugs available to inhibit the BTK, but BTK drugs are not specific. So we made it a very specific inhibitor, and we can use both the IKK and BTK inhibitors simultaneously. In one hand, you can inhibit tumor growth, and another one you can proliferate, expand, T cells that will produce less cytokine and will be less exhausted and can be more effective. So we are really excited about this work.
Since we published our paper in Oncotarget, we have two other papers coming out. One in Frontier Immunology in another is in Science Immunology, and we hope we can grow it and make it a much better successful model. With this, I would like to thank, again, the people at Oncotarget. Anybody who wants to collaborate with us, wants to look at this work, please contact us. Please let us know your opinion, whether it’s negative, positive, and we as scientists, we are open to any criticism, any positive impacts that we can improve the life of people.
With that, thank you very much.
Click here to read the full research paper, published by Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.