Behind the Study is a series of transcribed videos from researchers elaborating on their recent oncology-focused studies published in Oncotarget. A new Behind the Study is released each Monday. Visit the Oncotarget YouTube channel for more insights from outstanding authors.
—
Dr. Pradeep Tanwar discusses his study published in Oncotarget in 2016, entitled, “Germ cell-specific sustained activation of Wnt signalling perturbs spermatogenesis in aged mice, possibly through non-coding RNAs.”
Speaker
Welcome to the Oncotarget YouTube channel. This interview is with Dr. Pradeep Tanwar in the School of Biomedical Sciences and Pharmacy, University of Newcastle, Callaghan in New South Wales, Australia. (He) is talking about a manuscript published in Volume 7, Issue 52 of Oncotarget, titled, “Germ Cell-Specific Sustained Activation of Wnt Signaling Perturbs Spermatogenesis in Aged Mice, Possibly Through Non-coding RNAs.”
Dr. Pradeep Tanwar
Hi there. I’m Pradeep Tanwar, group leader at University of Newcastle, New South Wales, Australia. I’m also a fellow with Australian Research Council and Cancer Institute in South Wales. My laboratory is interested in studying the role of Wnt/βcatenin Central Signaling in report to track cancers. And for this study, we were interested in understanding how Wnt signaling plays a role in testicular development and testicular cancer.
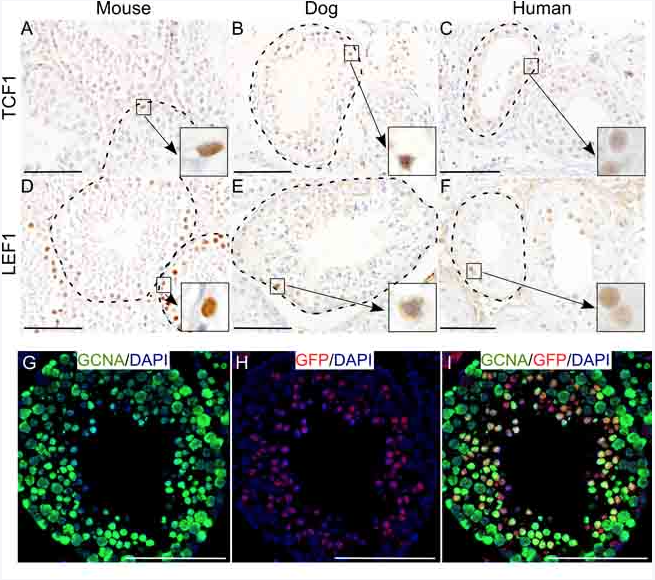
So we already know that the Wnt signaling or destruction or dysregulation of Wnt signaling is associated with human infertility and testicular cancer. So in this study, we examined whether Wnt signaling is really involved in this disease and how Wnt signaling contributes to this pathogenesis of this cancer. To do this, first we examined the expression of Wnt pathway membranes in mouse, dog, and human testes. And we found that Wnt signaling is quite active in spermatogonial stem cells compartment of all three tests. So then we wanted to understand how, when signaling is involved in spermatogenesis.
So we over-activated Wnt signaling in a mouse model in all the germ cells, including spermatogonial stem cells. And what we found was that, well, what activation had no effect on the young mice, but as they aged, we started finding defect in spermatogonial stem cell proliferation and differentiation, and that led to infertility.
We performed similar experiments on human cells, and we found that over-activation of Wnt signaling causes, defect, proliferation and differentiation germ cells. So next we wanted to know what was the reason for these defects. So we decided to perform RNA-Seq to look at the differential expression of the new data in non-mutated cells. And what we found that the majority of the genes, which we found were, that were differentially expressed, belong to a non-coding regions of the mouse genome. And then we looked at one of the non-coding RNA and that was a more significantly in the mutant compared to control.
So now next part of this study is to understand how this non-coding RNA regulates Wnt signaling and how that communication between these two pathways lead or affect spermatogenesis and lead to testicular cancer.
So currently what we are doing is we are first examining human testicular patient samples, and looking at the status of Wnt Signaling in these samples, also in the status of that non-coding RNA, which we have found.
And I think that will give us an information by how these two pathways interact or these two pathways contribute to the pathogenesis of human testicular cancer. And so, so far what we have found is that the Wnt signaling placed a core rule in spermatogonial proliferation and differentiation, or what activation of it causes defect in the spermatogenesis and in differentiation. And that lead to infertility.
Please feel free to subscribe to our YouTube channel and connect with us on Facebook, Twitter, or LinkedIn. Thank you.
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.

